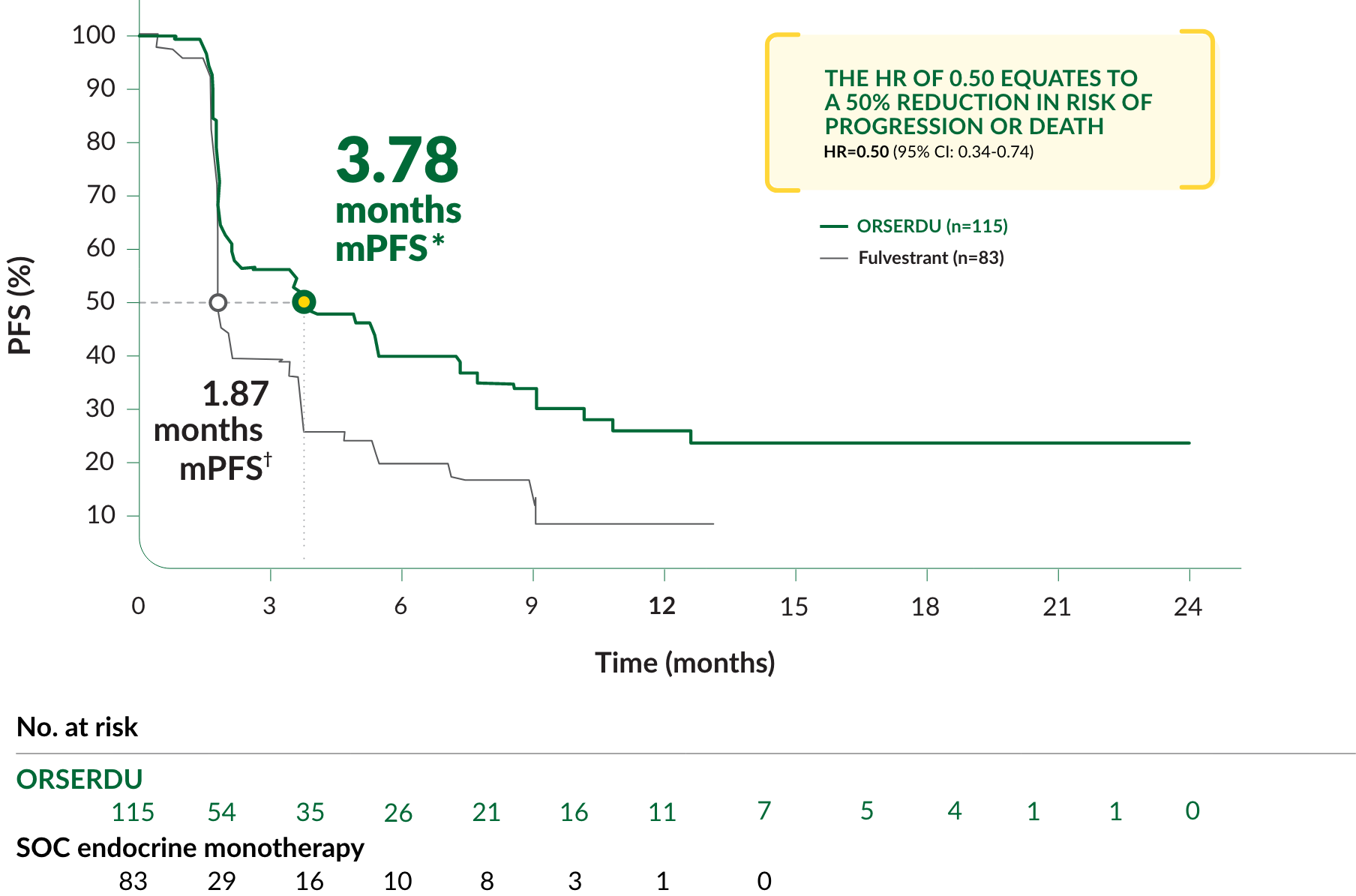
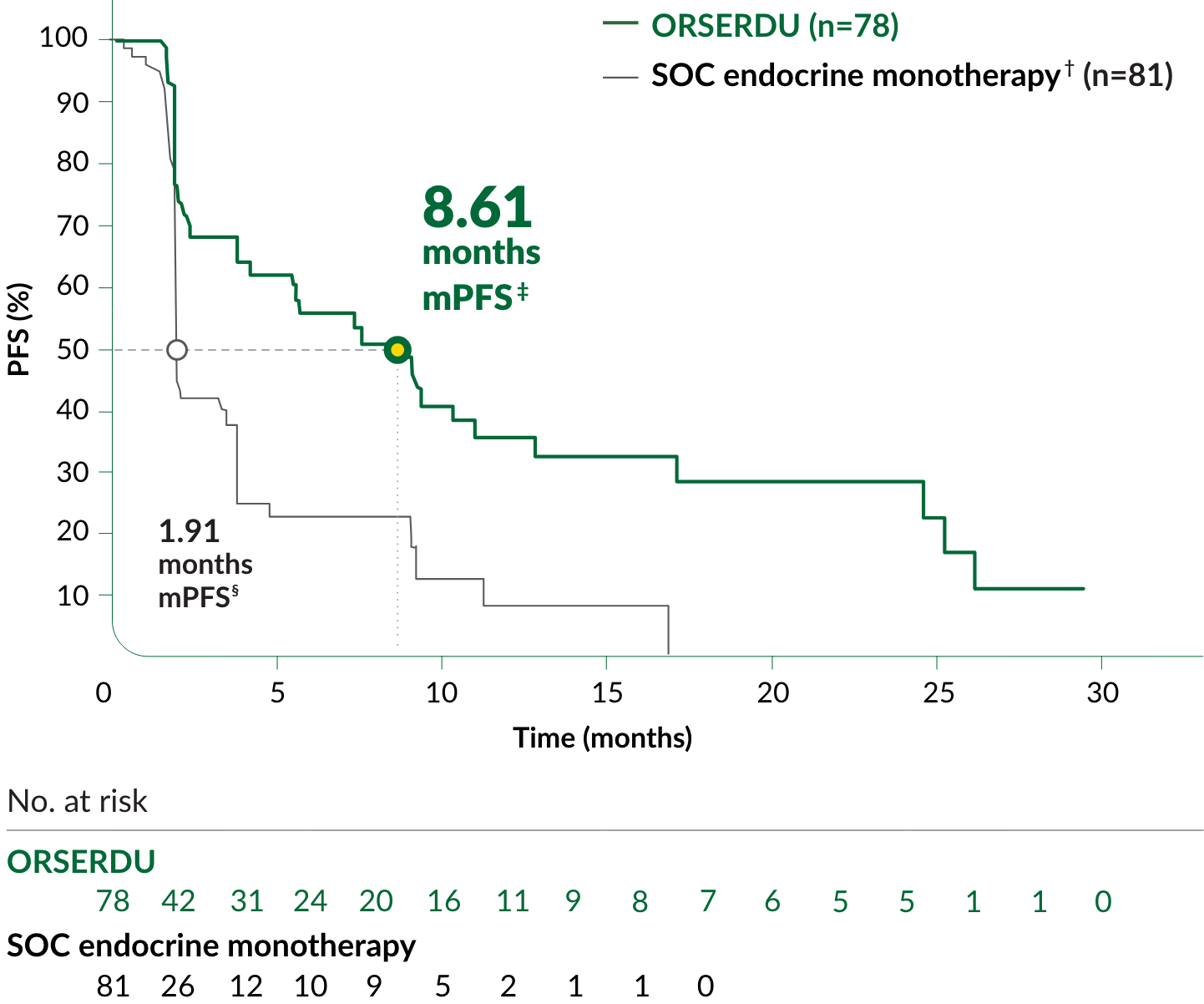
PRIMARY ENDPOINT IN EMERALD: PFS IN PATIENTS WITH ESR1-MUTATED mBC
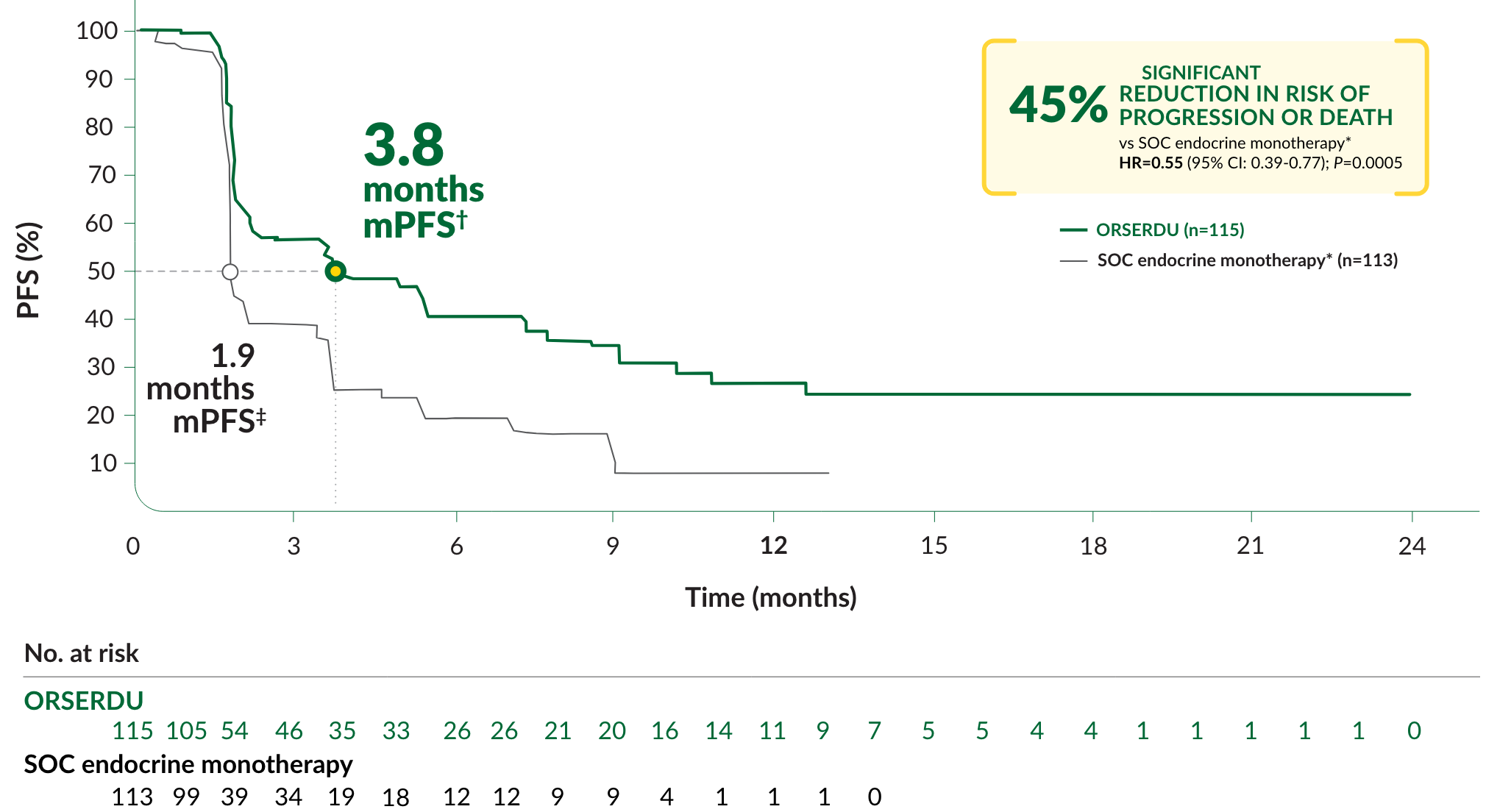
A statistically significant difference in PFS was observed in the ITT population and in the subgroup of patients with ESR1-mutated mBC. An exploratory analysis of PFS in the 250 (52%) patients without ESR1 mutations showed an HR of 0.86 (95% CI: 0.63, 1.19), indicating that the improvement in the ITT population was primarily attributed to the results seen in the ESR1-mutated mBC population
CI, confidence interval; HR, hazard ratio; ITT, intent to treat; mPFS, median progression-free survival.
*mPFS was the primary endpoint. SOC endocrine monotherapy included either fulvestrant, anastrozole, letrozole, or exemestane.
†95% CI: 2.2-7.3.
‡95% CI: 1.9-2.1.
Selected Important Safety Information
Warnings and Precautions
Dyslipidemia: Hypercholesterolemia and hypertriglyceridemia occurred in patients taking ORSERDU at an incidence of 30% and 27%, respectively. The incidence of Grade 3 and 4 hypercholesterolemia and hypertriglyceridemia were 0.9% and 2.2%, respectively. Monitor lipid profile prior to starting and periodically while taking ORSERDU.
Please see additional Important Safety Information below. Please see full Prescribing Information.